Athermal Plasticity and Field-Mediated Interfacial Bonding Anomalies¶
1. ABSTRACT¶
Standard Model Expectation: In a gravity-driven collapse ($\(U_g = mgh\)$ ), kinetic energy converts to deformation and heat. Material fusion requires thermal energy exceeding the melting point of the densest component. Structural steels soften and melt over a range (solidus/liquidus typically ($\(\sim 1400–1530^\circ\text{C}\)$ ), composition-dependent). Cellulose undergoes thermal decomposition/pyrolysis well below that range (onset ($\(\sim 200–300^\circ\text{C}\)$ ), with ignition dependent on oxygen, heat flux, moisture, and exposure time).
Empirical Contradiction: Forensic recovery of composite artifacts exhibiting the fusion of ferrous alloys with organic cellulose (paper) where the organic component remains distinct and unconsumed. Additionally, low-melting-point alloys (Zinc) are observed fused to high-melting-point alloys (Copper-Nickel) without liquid-phase separation.
Audit Objective: To evaluate whether the thermodynamic environment was thermal (fire-based) or interferometric (field-based).
2. CONTROL PARAMETERS¶
Thermodynamic System Definition: We treat the artifact as a Multi-Material Thermal Equilibrium problem.
-
The Pyrolysis Limit Constraint: Cellulose (Paper) begins irreversible thermal decomposition (Pyrolysis/Charring) at $\(T_{pyrolysis} \approx 300^\circ\text{C}\)$ .
Ferrous Alloys (Steel) undergo fusion/welding at $\(T_{melt} \approx 1538^\circ\text{C}\)$ . -
The "Thermal History" Veto:
- Standard Model: Thermal-history constraint (audit-safe): If a steel mass is at very high temperature for more than a brief interval, nearby cellulose at the interface would typically show charring/pyrolysis signatures, unless it is strongly shielded, oxygen-starved, or contact time is extremely brief.
- Constraint: If the paper interface is reported as uncharred/legible, the artifact is treated as inconsistent with sustained bulk heating of the surrounding ferrous mass to melting-range temperatures.
- Implication: Candidate mechanisms shift toward localized interfacial bonding / selective coupling without commensurate bulk-temperature equilibration (non-equilibrium / athermal in the bulk sense), rather than "phase change without ($\(\Delta T\)$)" as a literal claim.
Sintering Kinetics:
- Bulk Melting (Thermal): Homogeneous phase change. Low-melting alloys (Zinc) liquefy before High-melting alloys (Copper).
- Impedance-boundary bonding (field-coupled candidate): In conductor-selective coupling, power deposition can be localized at contacts/surfaces (frequency- and geometry-dependent), enabling interface bonding with less bulk soak-through than sustained external fire.
3. DATA CURATION & ANALYSIS¶
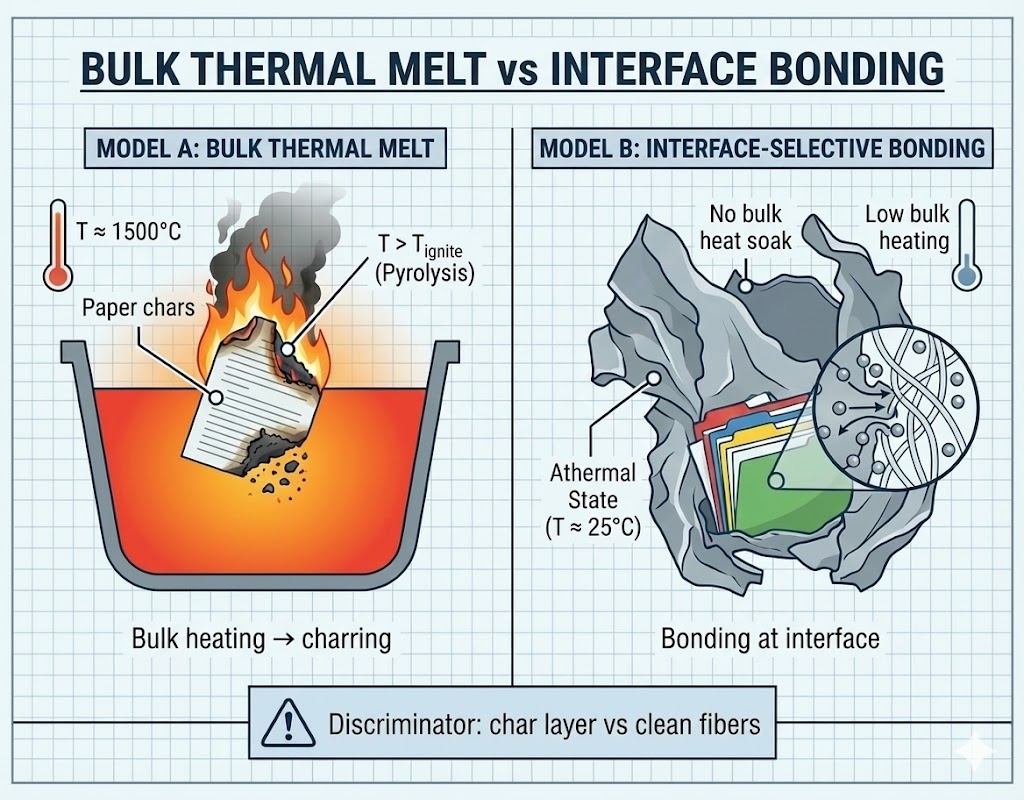
EVIDENCE FILE A: The Heterogeneous Composite (The Filing Cabinet and "The Meteorite")¶




- Visual Data: A high-density fused agglomeration consisting of resolidified ferrous metal (steel) and concrete aggregate. Embedded deep within the metallic matrix are stacks of paper documents. The paper fibers are visible, legible, and show no evidence of carbonization or ash formation.
- The Standard Model Defense: "Compression" or "Paper trapped in cool voids."
- Boundary Condition Violation:
- Radiant Transfer: Even if the paper was in a "void," a surrounding ferrous mass at melting-range temperature would generally impose large radiative/convective heat flux to adjacent cellulose, typically producing rapid pyrolysis/charring at the interface unless shielding/oxygen starvation/contact geometry strongly suppresses heat transfer. The key discriminator is interface morphology (char layer vs. clean cellulose fiber preservation).
- The Interface: The visual data shows paper fibers embedded in the matrix, not loose in a hole.
- Thermodynamic Veto: The survival of Legible Text (Ink/Cellulose) is difficult to reconcile with a uniform high-temperature thermal bath. It functions as a boundary condition supporting localized interface bonding / selective coupling without bulk thermal equilibration; interface chemistry and morphology (char layer vs preserved cellulose fibers) are the discriminator.
- Classification: Athermal interface bonding / localized impedance-boundary bonding (field-selective coupling; bulk-temperature equilibration not demonstrated).
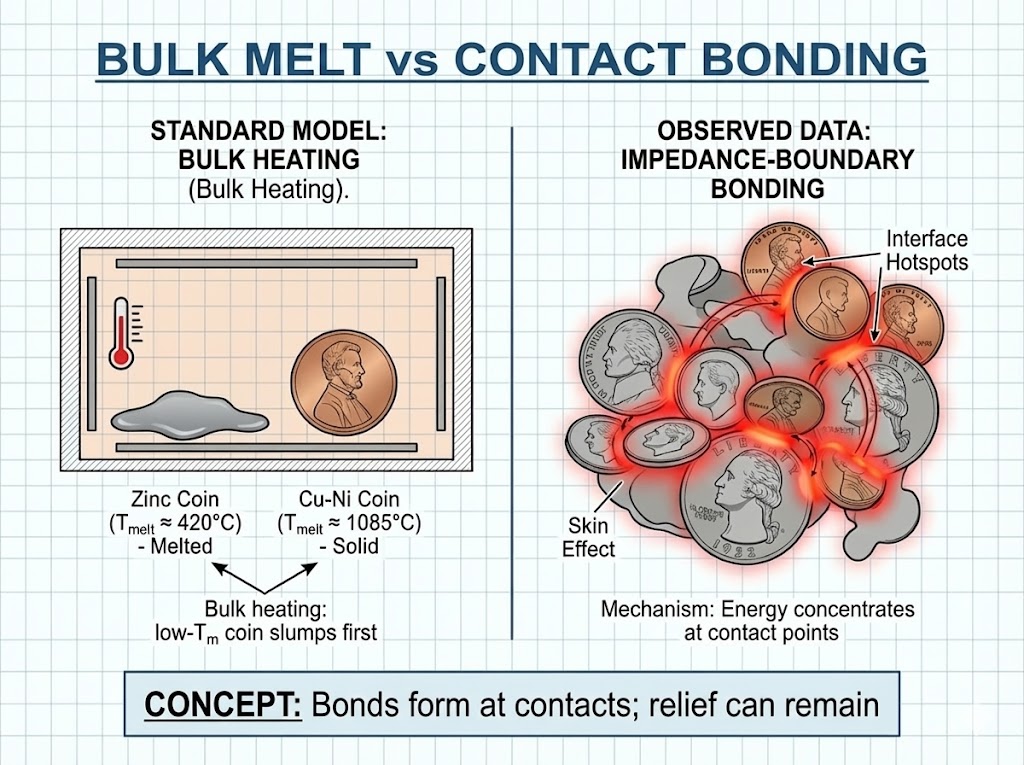
EVIDENCE FILE B: The Poly-Alloy Cluster ("Fused Coins")¶

- Visual Data: A fused cluster of U.S. coinage containing Zinc-rich pennies (Zn melt ($\(\sim 420^\circ\text{C})\)$ ) and Cu–Ni coinage (melting well above ($\(\sim 1100^\circ\text{C}\)$ ), alloy-dependent). The coins are sintered into a single mass but retain their individual minted relief and geometric integrity.
- The Standard Model Defense: "Brazing" (The Zinc melted and acted as glue).
- Boundary Condition Violation:
- Geometric Preservation: If the Zinc melted to act as a brazing filler, the Face Relief (Lincoln's profile) of the penny would be lost (slumped or dissolved).
- Observation: The Zinc coins retain their sharp geometric minting despite being fused to the higher-melting Copper.
- Mechanism: Interface bonding can occur via localized melting/softening of the lower-melting constituent at contact points while preserving much of the minted relief, depending on time/heat flux/constraint. Impedance-boundary / conductor-selective coupling is carried here as the leading explanation class if microscopy shows a ‘bond neck’ inconsistent with bulk flow/fillet meniscus typical of brazing.
- Classification: Impedance-boundary bonding / selective conductive coupling (conductor-regime label) — with brazing/partial-melt retained as competing thermal pathways, and microscopy used as the audit discriminator.
4. CORROBORATING BIO-TELEMETRY & SENSORY DATA¶
Objective: Cross-reference physical anomalies with independent human sensory inputs acting as biological transducers.
DATA SET A: Accelerated Oxidation Kinetics¶
Node-Recovery Zone [ID: Curator-01 | Calibration: Material Preservation Specialist]¶
Input Data: Subject intercepted compressed steel artifact ("File Cabinet").
Observation Specifics: Artifact appeared "messy and shiny" upon recovery but initiated aggressive oxidation ("rusting") within "two or three hours".
Boundary Condition: Reported accelerated oxidation kinetics post-recovery. This is carried as a lattice/chemistry instability phenotype, with controlled comparisons (humidity, wetting, salt contamination, cleaning agents, surface damage) used to bound conventional explanations for the rate.
CROSS-CALIBRATION: Corroborates Evidence File A (Lattice Instability).
DATA SET B: Conductor-Selective Particulate Emission¶
Node-West Broadway [ID: Video-Log-NIST | Calibration: Automated Optical Surveillance]¶
Input Data: Video telemetry of vehicle door handles prior to WTC 7 coming down.
Observation Specifics: Solid matter transitioning directly to aerosol/fume without a liquid phase.
Boundary Condition: Reported particulate emission without an obvious liquid-flow phase is treated as compatible with non-equilibrium surface loss / rapid oxidation / particulate generation. If attributed to field coupling, classify under conductor-selective coupling in conductive networks (CLC as default where loops exist; downstream $\(P=I2RP=I^2RP=I2R\)$), with IMD reserved for phenotypes implying bond-level decohesion/aerosolization rather than heat/oxidation alone.
CROSS-CALIBRATION: Corroborates Evidence File B (RF impedance-boundary coupling / conductor-regime coupling; CLC)
5. MECHANISMS OF NON-THERMAL FAILURE (Summary)¶
- Phenomenon: Paper Embedded in Steel $\(\rightarrow\)$ Mechanism: Athermal interface bonding (candidate) / impedance-boundary bonding (bulk thermal equilibration not shown).
- Phenomenon: Zn/Cu–Ni coin fusion $\(\rightarrow\)$ Mechanism: Impedance-boundary bonding / selective conductive coupling (conductor-regime label); retain brazing/partial-melt as competing pathway pending microscopy.
- Phenomenon: Rapid rusting $\(\rightarrow\)$ Mechanism: Reported accelerated oxidation kinetics (candidate lattice/chemistry instability phenotype; control for wetting/contamination).
6. FORENSIC MICROSCOPY PROTOCOL¶
Objective: To distinguish between Thermal Melting (Brazing/Flow) and Inductive Sintering (Diffusion).
TEST A: The Cellulose-Ferrous Interface Scan (The "Char" Test)¶
- Sample: Cross-section of the Paper/Steel interface.
- Standard Model Prediction (Thermal Trap):
- Chemistry: Carbonization Zone. Even if the paper survived, the interface layer must be pure Carbon (Char) due to radiant heat from the cooling steel.
- SCIE Prediction (Athermal Intercalation):
- SCIE-predicted discriminator : absence of a char/pyrolysis interlayer at the steel–cellulose interface, plus preservation of cellulose chemical signatures (e.g., via FTIR/Raman) adjacent to the bonded region. If metal infiltration is claimed, map it as morphological penetration (microfilaments/occlusion) rather than atomic intercalation unless high-resolution evidence supports it.
TEST B: The Coinage Alloy Distribution Map (The "Brazing" Test)¶
- Sample: Cross-section of the Zinc/Copper fusion neck.
- Standard Model Prediction (Brazing/Soldering):
- Morphology: Meniscus Fillet. The Zinc (or solder) should show liquid flow patterns (capillary action) filling the gaps between coins.
- Phase: The Zinc coin should be internally deformed or slumped.
- SCIE Prediction (Inductive Sintering):
- Morphology: Diffusion Bond. We look for a "Solid-State Weld" at the contact point where atoms migrated across the boundary, but the bulk Zinc retained its Grain Structure (Unmelted).
- Implication: Energy was deposited strictly at the Impedance Boundary (Skin Depth), consistent with RF coupling.
7. SYNTHESIS: The SCIE Classification Protocol¶
Thermodynamic Gap: A purely thermal pathway that melts/strongly fuses ferrous material would normally produce collateral cellulose pyrolysis/charring at adjacent interfaces unless extraordinary shielding/oxygen starvation is demonstrated.
Circuit Gap: Coin/metal bonding with preserved relief motivates a localized interface-energy deposition hypothesis (selective conductive coupling / impedance-boundary bonding under a conductor-regime label) if microscopy rules out bulk-flow brazing signatures.
The Classification:
- Rule A (Attributes): The event exhibits (1) Athermal Fusion (Meteorite), (2) Material Selectivity (Zn vs Cu), and (3) Lattice Instability (Rapid Oxidation).
- Rule B (Justification): These artifacts are carried as material-selective bonding phenotypes that are difficult to square with a uniform high-temperature thermal history; within this dossier, a SCIE-class coupling framework is advanced as the leading explanation class, with interface chemistry/morphology tests serving as the audit discriminator.